LONDON — The rapidly spreading monkeypox outbreak constitutes a global health emergency, the World Health Organization’s highest alert level, WHO director-general Tedros Adhanom Ghebreyesus declared on Saturday.
First identified in monkeys, the virus is transmitted chiefly through close contact with an infected person. Until this year, the viral disease has rarely spread outside Africa where it is endemic.
But reports of a handful of cases in the United Kingdom in early May signalled that the outbreak had moved into Europe.
Cases have since ballooned to more than 16,000 from than 75 countries. Five deaths, all of which occurred in Africa, have been reported so far.
RELATED: WHO declares global health emergency over monkeypox outbreak
How dangerous is it?
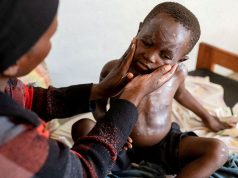
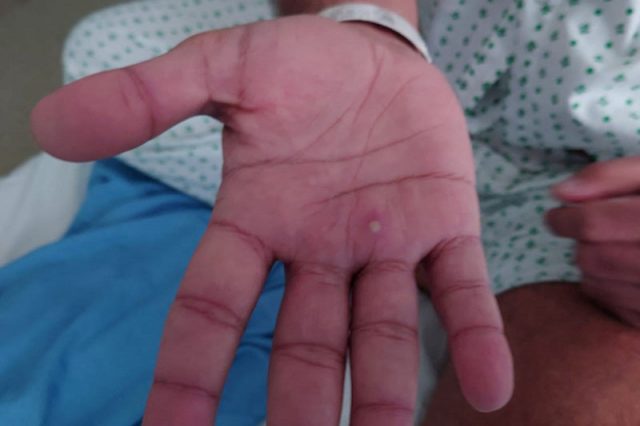
Monkeypox is a virus than typically causes mild symptoms including fever, aches and pus-filled skin lesions. People tend to recover within two to four weeks, according to the WHO.
Anyone can spread the virus, but the current outbreak outside of Africa is concentrated almost exclusively among men who have sex with men.
Monkeypox spreads primarily via intimate skin-to-skin contact, usually with someone who has an active rash, as well as via contact with contaminated clothes or bedding. It is not as easily transmitted as the SARS-CoV-2 virus that spurred the COVID-19 pandemic.
“COVID is spread by respiratory route and is highly infectious. This doesn’t appear to be the case with the monkeypox,” said Dr. Martin Hirsch of Massachusetts General Hospital.
The risk of monkeypox is moderate globally, except in the Europe, where the WHO has deemed the risk as high.
Why is it an emergency?
Health officials from several countries had urged the WHO to label monkeypox a public health emergency of international concern due to the quick escalation of cases and concerns it may become endemic in more countries.
The emergency declaration aims to spur global action and collaboration on everything from testing to the production and distribution of vaccines and treatments.
The fatality rate in preceding monkeypox outbreaks in Africa of the strain currently spreading has been around 1%, but so far this outbreak appears to be less lethal in the non-endemic countries, many of which have stronger healthcare infrastructure.
Scientists are trying to determine what caused the initial spate of cases and whether anything about the virus has changed. Increased global travel as well as climate change have generally accelerated the emergence and spread of viruses, experts say.
Infectious disease experts say that years of financial neglect has left sexual health clinics—who are on the frontline of the current monkeypox response—ill-prepared to curb further spread.
Protective measures
Health officials say that people should avoid close personal contact with someone who has an illness presenting with a distinctive rash or who is otherwise unwell. People who suspect they have monkeypox should isolate and seek medical care.
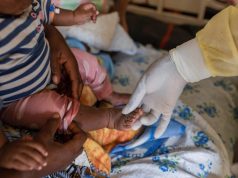
Health officials have also been offering monkeypox vaccines to high-risk individuals and those that have recently been in close contact with an infected person.
Danish company Bavarian Nordic has a vaccine that goes by the brand name Jynneos, Imvamune or Imvanex—depending on geography. It has U.S. and European approval for the prevention of both smallpox and the closely related monkeypox.
An older vaccine, currently made by Emergent Biosolutions, is called ACAM2000, but its uptake has been limited due to a severe side-effect warning.
Bavarian Nordic says it can produce 30 million doses of its vaccines—including the monkeypox one—each year, and has tapped a U.S.-based contract manufacturer to increase monkeypox vaccine capacity.
Treatment
Monkeypox symptoms often resolve on their own within weeks. Patients may receive extra fluids and additional treatment for secondary bacterial infections.
An antiviral agent called tecovirimat—branded as TPOXX and made by SIGA Technologies—has U.S. and EU approval for smallpox, while its European approval also includes monkeypox and cowpox.
— Reporting by Natalie Grover in London and Michael Erman in New Jersey; Editing by Michele Gershberg, William Maclean