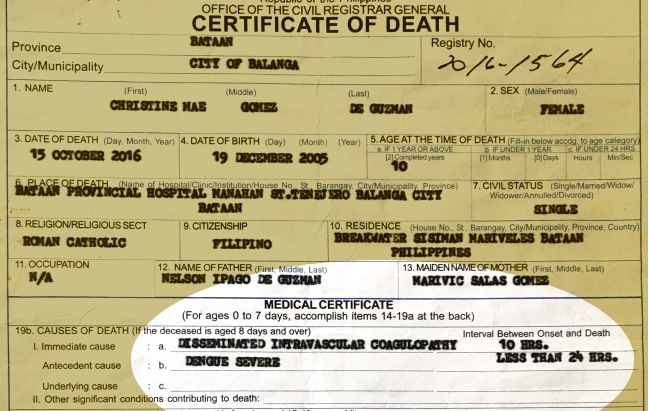
(UPDATED – 6:03 p.m.) MANILA, Philippines — The Food and Drug Administration (FDA) of the Philippines has ordered French pharmaceutical giant Sanofi Pasteur to stop the sale and distribution of Dengvaxia “to protect the general public” from health risks associated with the world’s first anti-dengue vaccine, even as a death certificate indicated that a girl in Bataan, identified as Christine Gomez de Guzman, had died of dengue after receiving the initial dose of the vaccine.
FDA Advisory No. 2017-318 “immediately directed Sanofi to SUSPEND the sale/distribution/marketing of Dengvaxia and cause the WITHDRAWAL of Dengvaxia in the market pending compliance with the directives of” the agency.
“Sanofi was further directed to conduct an information dissemination campaign through Advisories, Dear Doctor Letters and Patient fora,” the FDA further noted in its advisory.
In an advisory issued last November 29, Sanofi said that based on post-clinical trial study, it was found that Dengvaxia posed potential risk to patients who have not had dengue prior to immunization.
The FDA said it is now “closely coordinating” with the Department of Health “for any adverse events/ reactions that may be reported by the recipients following their immunization of the Dengvaxia, and will immediately take appropriate measures to protect the public.”

“All drug establishments, including consumers and non-consumer user (e.g. healthcare professionals) are enjoined to take part in the post marketing surveillance of Dengvaxia, by reporting to FDA any incident that reasonably indicates that Dengvaxia has caused or contributed to the death, serious illness, or serious injury to a consumer, a patient, or any person,” the agency said.
In response to the FDA, Sanofi Pasteur said it will “work with them to review the implementation of their direction.”
SANOFI: CONSTRUCTIVE, TRANSPARENT DIALOGUE
In a press release, Sanofi Pasteur added, “We will continue to seek constructive and transparent dialogue with them.”
It also issued a clarification about the safety of the vaccine, saying Dengvaxia “does not contain any viruses that can make people ill with dengue or severe dengue.”
“If you have had no previous dengue infection before vaccination, the vaccine does not give you dengue,” Sanofi Pasteur said.
“The increased risk identified from the new analysis translated to two additional cases of ‘severe dengue’ out of 1,000 previously dengue-uninfected people vaccinated over five years of follow-up. In this group, all fully recovered with proper medical treatment,” it said.
In a press conference on Monday, Sanofi Pasteur regional director Dr. Joselito Sta. Ana explained their definition of “severe” dengue:
“Ang iniisip kasi natin na kapag severe, ito na ‘yung dengue shock na mamamatay. Hindi… Ang severe could only be ‘yung lagnat ng dalawang araw, at kapagka may suspect na case ng dengue, ‘yung platelet count pwede bumaba. Pwede ring kapag nabunggo ka, magkaka-hematoma ka o magkakapasa ka. At pwede ring parang pagka nainitan ka, mababalinguyngoy ka (When we think about severe, we associate it with deadly dengue shock. But that’s not the case… Severe here could only be fever for two days, and if there’s a suspected case of dengue, the platelet count could go down. It could also be that if you bump into something, you get a hematoma or bruises. And if it gets hot, you could get a nosebleed),” he said.
Meanwhile, the committee on good government and the committee on health at the House of Representatives will reopen their inquiry into the government’s immunization program using Sanofi’s Dengvaxia.
Surigao del Sur Rep. Johnny Pimentel, chairperson of the committee on good government, announced a hearing on December 13 at 1:30 p.m.
“We are reopening the dengue case because of recent issues,” he said.
Asked about the findings of the good government committee, Pimentel said, “There were some lapses in the procurement, but the Department of Health certified it and Sanofi said it was safe to use the vaccines.”