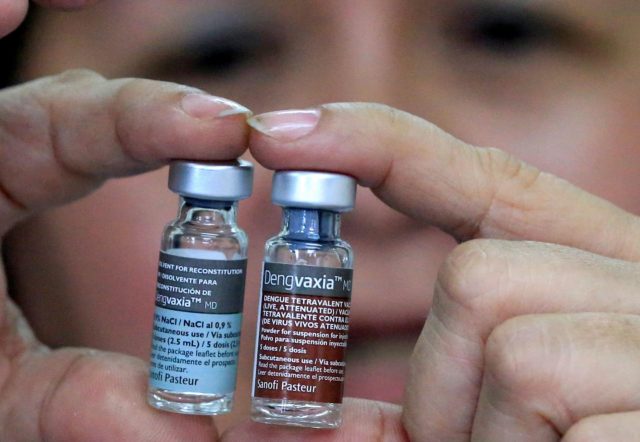
MANILA – The Philippines has fined Sanofi P100,000 and suspended clearance for the French drug maker’s controversial dengue vaccine Dengvaxia, citing violations on product registration and marketing, its health secretary said on Thursday.
Concerns over the dengue immunization of nearly 734,000 children aged nine and above resulted in two congressional inquiries and a criminal investigation as to how the danger to public health came about.
Sanofi was ordered to stop the sale, distribution and marketing of Dengvaxia after the company last month warned the vaccine could worsen the disease in some cases.
“They were fined and their certificate of product registration was suspended,” Health Secretary Francisco T. Duque III told Reuters.
The Food and Drugs Administration found Sanofi violating post-marketing surveillance requirements, he added.
SANOFI’S COMMENT
On Friday, Sanofi Pasteur confirmed it received an FDA notice of its one-year suspension of its Dengvaxia Marketing Authorization. “According to the notification, this suspension is linked to an alleged failure to comply with post-marketing requirements and is not linked to the product profile,” the pharmaceutical firm said in a statement.
“As part of our standard company practices, Sanofi routinely conducts post-approval commitments to continuously evaluate the safety and effectiveness of our vaccines in the countries where they are in use, and we have done so in the Philippines with Dengvaxia. Sanofi Pasteur confirms that in accordance with international and local laws, regulations and company standards, post-approval commitments for Dengvaxia, as described in the pharmacovigilance plan submitted to the Philippines FDA and other national regulatory authorities, have been – and will continue to be – fulfilled,” it added.
Sanofi Pasteur vowed to “continue to cooperate in full transparency with the Philippines FDA” and said it “is committed to comply with the Philippines laws and regulations.”
According to Sanofi, “the global risk management and pharmacovigilance plan for Dengvaxia is the same for all countries where the product has been approved, and periodic reports are submitted to the national regulatory authorities (NRAs) where Dengvaxia is approved. No other requests of market withdrawal or license suspension have been received from other NRAs where the vaccine is approved.”
The government spent P3.5 billion ($70.2 million) for the Dengvaxia public immunization program in 2016 to reduce the 200,000 dengue cases reported every year.
The timeline of approvals and processing of the decision by the administration of then President Benigno Aquino III in late 2015, just a few months shy of the May 2016 elections, drew observations that it was done with “undue haste” and a certain “recklessness” despite warnings raised by some medical quarters against rolling out the vaccine at such a massive scale given its newness.
Several of the experts who had expressed reservation testified at last month’s Senate hearings that were opened after Sanofi issued a Nov. 29 advisory that further review of clinical studies indicated that while Dengvaxia is effective against preventing repeat infections of dengue, injecting it on seronegative people or those who never had prior infections could expose them to risk of “severe disease” or a powerful strain of dengue.
During Senate hearings, some Blue Ribbon probers expressed the view the haste may have been impelled by a political agenda by the Aquino administration, as the ruling Liberal Party was fielding candidates in the May 2016 elections.
However, former President Aquino, testifying at the Blue Ribbon inquiry, insisted he was solely motivated by the desire to cut what he described as the alarming incidence of dengue.