The country’s Food and Drug Administration advised the public against the purchase of four pediatric drug products found containing dangerous contaminants.
This advisory was issued following the recent deaths of over 100 children in India and The Gambia due to acute kidney failures that are linked to these cough syrup medicines.
The FDA also mentioned in its post that the products were detected in the African region last September.
“The Food and Drug Administration (FDA) notifies the public on the WHO Medical Product Alert on four substandard (contaminated) pediatric drug products which were detected in the African region in September 2022,” the state regulator said.
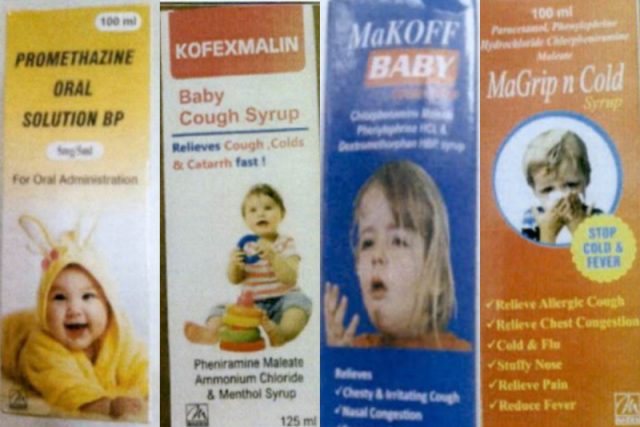
The FDA also accompanied its post with a screenshot that showed images of these drugs.
The following are the products that have been flagged by the World Health Organization as substandard (contaminated):
- Promethazine Oral Solution BP
- Kofexmalin Baby Cough Syrup
- MaKOFF Baby Cough Syrup
- MaGrip n Cold Syrup
It was indicated that all four items are manufactured by Maiden Pharmaceuticals Ltd., an Indian pharmaceutical company.
In a statement, the state regulators noted that these medicines contained dangerous contaminants that can cause different conditions which may lead to death.
“The FDA strongly advises the public to be vigilant on the circulation of these substandard drug products since its contaminants, Diethylene Glycol and Ethylene Glycol, are toxic to humans when consumed and may result in abdominal pain, vomiting, diarrhea, inability to pass urine, headache, altered mental state and acute kidney injury which may lead to death,” the FDA said.
The agency said that these drug products are not registered.
The FDA also requested all local government units and law enforcement agencies to make sure these are not sold or administered to patients in any localities in the country.
Anyone who has inquiries or more information about the abovementioned items can email FDA at [email protected].
Meanwhile, the unauthorized sale and distribution of these syrups can be reported to this email address: [email protected].
Banned in Indonesia
International reports said that Indonesia banned the sale of all four Indian-made cough syrups amid the nationwide investigation of the deaths of at least 99 children due to acute kidney injuries.
Ingredients of these liquid products were linked to their cause of death.
Al Jazeera reported on October 24 a story about an Indonesian child who had died after her mother gave her flu medicine because she had a cough.
The report said that this case was among the other tragic incidents being investigated by the Indonesian government.
‘All her organs were damaged – her brain, heart, liver, and eyes. On Sunday morning, she had heart failure. She died in my lap.’
At least 133 Indonesian children have died from acute kidney injured, linked to medicinal syrups. The death toll is expected to rise. pic.twitter.com/JYONNkwJDN
— Jessica Washington (@JesWashington) October 24, 2022
The Gambia, a country in West Africa, also logged 70 child deaths that are linked to these medicines manufactured by the same pharmaceutical company.
On October 5, the WHO released an analysis of these pharmaceutical items.
The organization detected diethylene glycol and ethylene glycol in them. These compounds are toxic to humans when consumed.
“All batches of these products should be considered unsafe until they can be analyzed by the relevant National Regulatory Authorities. The substandard products referenced in this alert are unsafe and their use, especially in children, may result in serious injury or death,” WHO said.









