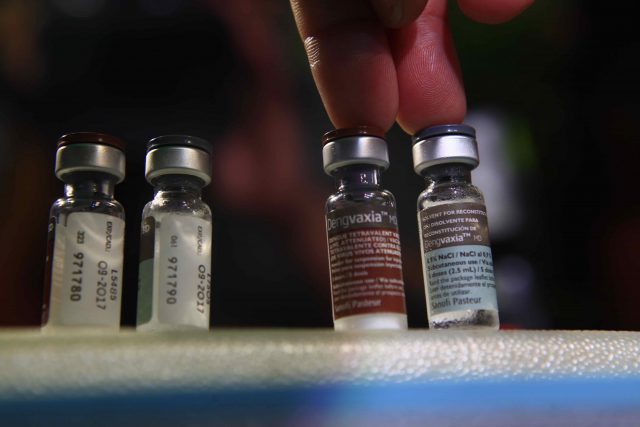
MANILA – The Food and Drug Administration (FDA) recently ordered pharmaceutical company Sanofi and drugstore chain Watsons Personal Care Stores to stop their immunization activities in Watsons stores, saying it had not authorized them to conduct these.
FDA Director General Nela Charade Puno said that because of the lack of authorization, the public was not assured of the “safety and quality of the service” in the stores. The immunization activities, she added, posed “a potential health hazard” to consumers.
The FDA ordered the two to “show cause why administrative and criminal charges should not be filed against them for conducting such activities without FDA authorization.”
For its part, Sanofi said it worked within the laws and regulations governing the conduct of pharmaceutical companies, and was taking part in the FDA’s investigation into the matter. It added that it was taking the issue “seriously”.
The pharmaceutical company is the license-holder of dengue vaccine Dengvaxia.
In March, philstar.com reported that over 100 Watsons stores were scheduled to conduct in-store Dengvaxia vaccination in April, October, and April 2018 for the first, second, and third doses, respectively.
The Department of Health had previously assured the public of the drug’s safety and efficacy. In fact, it had even rolled out the dengue immunization program, using Dengvaxia, for public school students in April last year.
Sanofi revealed that it had received a summons from the FDA, and so did Watsons.
“We have complied with the required submission of our answer and, in deference to the proceedings before the FDA, we are refraining from making further comments,” Sanofi said in its statement.
“Laws are mandatory and they are meant to be followed,” Puno said. “Compliance to the law is not optional, especially when the safety and health of the public are involved. Sanofi, Watson’s, and other manufacturers and drugstores are hereby warned. Nobody is exempt.”
InterAksyon is still seeking Watsons’ statement on the matter, although it had announced through its Facebook page on April 27 that it was postponing the scheduled vaccinations for dengue, pneumonia, and cervical cancer.
It said that the FDA had issued a directive on April 25 for drugstores to apply for a special permit to conduct in-store vaccinations, and that it was now applying for this permit.