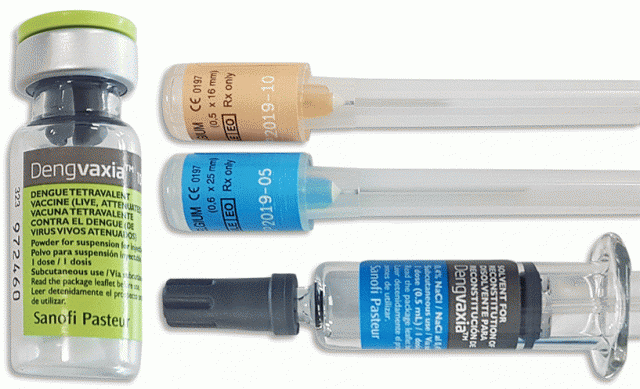
In a public disclosure dated November 29 in Paris, the pharmaceutical company Sanofi disclosed that a new analysis of long-term Dengvaxia® data found differences in vaccine performance based on whether or not the vaccine subject has had prior dengue infection.
According to Sanofi’s disclosure, there are many factors that can lead to severe dengue infection. “However, the highest risk of getting more severe disease has been observed in people infected for the second time by a different dengue virus … For individuals who have not been previously infected by dengue virus, vaccination should not be recommended.”
This belated finding is of no small consequence in the Philippines, as the Department of Health’s Dengue School-based Immunization Program had already started, during the term of then health secretary Janette Garin, in the school year 2015-2016 covering hundreds of thousands of public school pupils nine years and older enrolled in Grade IV, and administered in three six-month interval doses up to June 2016.
The great majority of those pupils have certainly not had been previously infected by dengue.


Dr. Susan Pineda-Mercado, former DOH undersecretary and chief of staff and international public health expert and communication strategist, remarked in a Facebook post: “This is the biggest government funded clinical-trial-masked-as-a-public-health-program scam of an experimental drug in the history of the DOH.”
Dr. Mercado observed that the program cost the Philippines PhP3 billion for the vaccines “and so much more now for the risks, anxiety and even lives that are now endangered.
“This was reckless and irresponsible from the start and the public was deceived into thinking this vaccine would protect children from dengue.
“The public health community has been outraged for over a year. Legal action is now necessary. Families should be compensated for damages and the decision-makers behind this deal should be brought to justice.
“We need a core group of lawyers for a legal strategy. As several countries are involved multiple organizations for the protection of children should join forces.”
According to a FAQ sheet published online by DOH, the vaccine is aimed at protecting against dengue caused by dengue virus serotypes 1, 2, 3 and 4. It is administered via subcutaneous injection in the upper arm (deltoid).
Meanwhile, Sanofi has stated it will ask regulators to update product label to the reflect new information, which states, in part: “Based on up to six years of clinical data, the new analysis evaluated long-term safety and efficacy of Dengvaxia in people who had been infected with dengue prior to vaccination and those who had not. The analysis confirmed that Dengvaxia provides persistent protective benefit against dengue fever in those who had prior infection. For those not previously infected by dengue virus, however, the analysis found that in the longer term, more cases of severe disease could occur following vaccination upon a subsequent dengue infection.”
Click here to read the online disclosure of Sanofi: Sanofi updates information on dengue vaccineM